The Annual Meeting of the American Association for Cancer Research (AACR), a global event for basic and translational study on cancer, was grandly held in Orlando, Florida, USA from April 14 to April 19, 2023. The Annual Meeting is the 114th Annual Meeting of AACR. At the meeting, Suzhou Puhe Biopharma submitted two independently developed projects, PH009-1 (the fourth-generation EGFR project) and PH020-803 (a new generation PRMT5 inhibitor), to the AACR Annual Meeting. This is also the first time that the study data of Puhe Biopharma has been publicly disclosed, and both projects have been highly recognized by the Meeting committee. Where, PH009-1 was presented with posters at the Meeting, PH020-803 was invited to give an oral presentation at the meeting. Currently, both abstracts have been made public online.
I Oral report: A new generation of MTA synergistic and brain permeable highly selective PRMT5 inhibitors

PRMT5 is a classic target of epigenetics, using SAM as a methyl donor to mediate arginine symmetric dimethylation of protein substrates. PRMT5 is overexpressed in many types of tumors, and its expression level is negatively correlated with patient prognosis, has long being an important target for tumor therapeutic drugs study. However, PRMT5 is crucial for the hematopoietic system, and hematotoxicity is a major challenge in the development of PRMT5 inhibitors. Among the PRMT5 inhibitors under clinical study, substrate competitive inhibitors (such as GSK3326595) or SAM competitive inhibitors (such as JNJ64619178) can both exhibit dose limited hematotoxicity in clinical studies; Hematotoxicity limits the increase in dosage, leading to unsatisfactory clinical efficacy. However, if we can selectively choose to inhibit the PRMT5 activity in tumor cells without affecting the PRMT5 activity in normal cells, we may avoid hematotoxicity and improve clinical efficacy. In recent years, researchers have discovered a synthetic lethal mechanism between PRMT5 inhibition and MTAP deficiency, providing feasibility for selectively PRMT5 in targeting tumor cells. MTAP deficiency has a high incidence in various malignant tumors; As the only metabolic enzyme of MTA in the body, MTAP deficiency can lead to a large accumulation of MTA in cells. They have similar affinity and binding sites to PRMT5 due to the structural similarity between MTA and SAM. The accumulation of MTA in tumor cells can compete with SAM, leading to partial inhibition of PRMT5 activity. Therefore, compared with wild-type normal cells, tumor cells with MTAP deficiency are more sensitive to PRMT5 activity inhibition, generating a certain selective safety window. To further expand this safety window, small molecules can selectively stabilize the binding of PRMT5 to MTA, on combination of the subtle differences in the structures of MTA and SAM, but affecting the binding of PRMT5 and SAM, thereby further expanding the safety window. Currently, Amgen and Mirati and some other companies are developing MTA synergistic PRMT5 inhibitors, and Puhe Biopharma PH020-803 is also developing a new generation of PRMT5 inhibitors under similar strategies.
In tumor cells with MTAP deficiency, 803 strongly inhibits SDMA production and cell growth, but has very weak activity against wild-type cells. Comparing to the 3-4 fold cell selectivity of GSK3326595 and JNJ64619178, the cell selectivity of 803 reaches over 80 times, and it has shown good blood safety in artificial blood stem cell studies and high-dose repeated administration studies in mice, significantly better than GSK3326595. Meanwhile, during the activity detection of 44 methyltransferases, 803 strongly inhibited PRMT5 and its mutants without significant effect on the activity of other methyltransferases. 803 showed good pharmacokinetic properties and good oral absorption in both rats and dogs, and its intravenous administration in rats showed its high brain penetration potential. In the in vivo pharmacological trials, 803 showed strong tumor growth inhibition in mouse tumor models of different MTAP deficiency. Generally, the data shows that 803 is a highly selective PRMT5 inhibitor with MTA synergy and brain permeability, which can be used for the treatment of malignant tumors with MTAP deficiency. Due to the high incidence of MTAP deficiency in malignant tumors, 803 has enormous market potential in the anti-tumor field.
II. Poster display: the fourth-generation EGFR inhibitors that can overcome all common and drug-resistant mutations

In NSCLC, 10-50% of patients have EGFR activated mutations (Asia: 49.1%; Europe: 12.8%). Where, the common EGFR Ex19del and L858R Ex21 mutation, are sensitive to first-generation and second-generation EGFR inhibitors, such as gefitinib, erlotinib, and afatinib, which can be used for treatment. However, patients often develop new mutations after treatment, namely T790M mutations, leading to drug resistance and disease progression. Although the third-generation EGFR inhibitor Osimertinib can effectively inhibit the T790M mutation, 10-24% of patients develop C797S resistance mutations after Osimertinib treatment. The development of a fourth-generation EGFR inhibitor that can overcome the C797S mutation is urgently needed in clinical practice. See the table below for details.
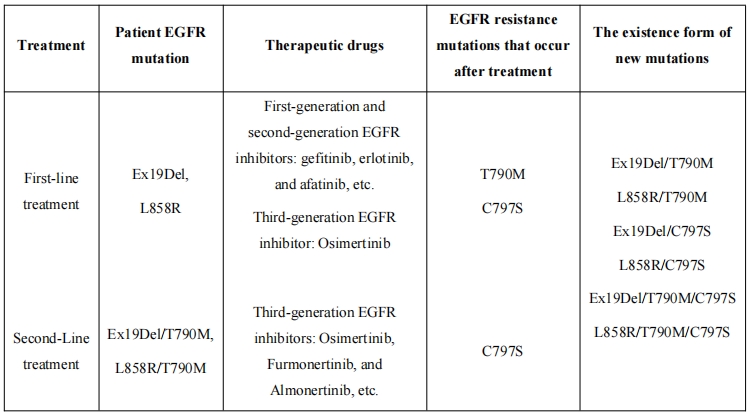
The fourth-generation EGFR inhibitor PH009-1 of Puhe Biopharma exhibits strong inhibitory activity against all single, double, and triple mutations (with IC50 less than 4 nM for all mutated Baf3 cells), maintaining good selectivity towards wild-type EGFR cells; Both kinase profiling and Safety Panel studies have shown an extremely low off-target risk for PH009-1. Meanwhile, preclinical data shows that PH009-1 has good potential for brain penetration. In EGFR single, double, and triple mutation tumor efficacy mouse models, PH009-1 showed strong anti-tumor activity, leading to the long-term regression of tumors. In the tumor efficacy model of Ex19Del single mutation, there was no tumor rebound, no effect on weight gain, and no animal death observed after more than six months of administration; at the end of administration, the weight gain of mice in the positive control group of Osimertinib group was lower than that in the PH009-1 group, and animal death occurred. These data confirm the excellent selectivity, effectiveness, and long-term medication safety of PH009-1, as well as its great potential to become a first or second line treatment. Currently, many fourth-generation EGFR inhibitors are under study both domestically and internationally. In order to achieve long-term control of tumors, it is best for fourth-generation molecules to overcome common Ex19Del and L858R mutations as well as T790M drug-resistant mutations while overcoming C797S mutations, achieving full line control of EGFR mutations. However, these requirements greatly increase the difficulty of molecular R&D. Currently, the clinical progress on the fourth-generation EGFR inhibitors under study is not ideal due to off target toxicity, poor selectivity towards wild-type EGFR, or molecular toxicity issues. Although BLU945 has made good clinical progress internationally and is effective against triple mutations containing C797S, it has weaker activity against certain single or double mutations without T790M mutations, such as Ex19Del and Ex19Del/C797S. This means that BLU945 needs to be used in combination with other molecules such as BLU525 in clinical practice.
About Suzhou Puhe Biopharma Co., Ltd.
Puhe Biopharma is a small molecule innovative pharmaceutical company jointly funded and incubated by Shiyu Capital and well-known domestic listed pharmaceutical companies. Our focus is on developing groundbreaking drugs in the field of oncology and supportive cancer care, committed to addressing distinct clinical needs. We adopt a multifaceted approach that blends scientific insight, financial acumen, and industrial strategy, alongside a market-oriented, risk-balanced operational method.
Leveraging our domestic clinical strengths through strategies such as company mergers and acquisitions, product introductions, and independent R&D, Puhe Biopharma actively explores commercial opportunities. We capitalize on the benefits of flexible production scale and comparatively low-cost investments, integrating resources to expedite our industrial growth. In 2022, our innovative approach earned us a spot in the Jiangsu Province Science and Technology Small and Medium-Sized Enterprise Database.
Currently, Puhe Biopharma has an impressive portfolio of small molecule drugs for cancer and supportive therapies, targeting unmet clinical needs in lung and pancreatic cancer, among others. A standout in our product pipeline is YK-029A, a leading and rapidly advancing innovative drug. Having received acceptance and approval from the National Medical Products Administration, YK-029A is now progressing into the pivotal Phase III of clinical trials.