Recently, the first clinical study result of the Class I innovative drug YK-029A, originally developed by Puhe Biopharma, was included in the 2023 ASCO poster discussion. The poster presentation and on-site poster discussion session were held on June 4 in the United States time. Professor Duan Jianchun, from Professor Wang Jie's team at the Cancer Hospital of the Chinese Academy of Medical Sciences, attended and gave a keynote presentation.
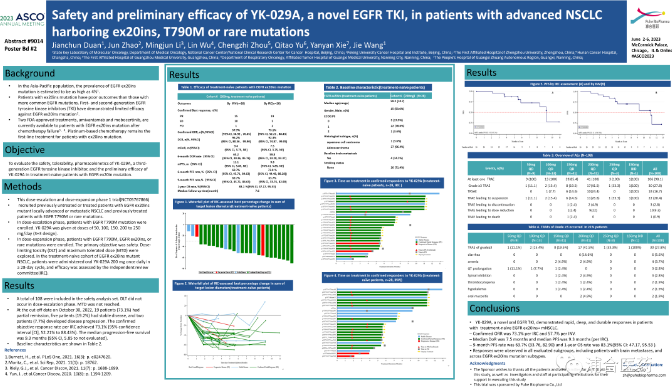
Abstract Title: YK-029A, a novel EGFR tyrosine kinase inhibitor, safety and preliminary efficacy in advanced NSCLC patients carrying EGFR Ex20ins, T790M, or rare mutations. Safety and preliminary efficacy of YK-029A, a novel EGFR TKI, in patients with advanced NSCLC harboring Ex20ins, T790M or rare mutations.
Reported by: Professor Duan Jianchun, Cancer Hospital of the Chinese Academy of Medical Sciences
Summary number: 9014
This study is a national multicenter clinical Phase I study led by Professor Wang Jie's team from the Cancer Hospital of the Chinese Academy of Medical Sciences. In the Phase I study, Professor Wang Jie's team conducted a trial design of "3+3" dose escalation (Ia) and multi queue dose expansion (Ib), and the results showed that YK-029A exhibited good efficacy and safety against mutations such as EGFR T790M and EGFR Ex20ins in advanced NSCLC at lower dose levels.
Efficacy analysis: In the initial treatment cohort carrying EGFR Ex20ins mutation, 26 patients were included in the efficacy analysis set. As of October 30, 2022, 19 patients (73.1%) achieved partial remission (PR), 5 patients (19.2%) were in stable condition (SD), and 2 patients (7.7%) experienced disease progression (PD). The objective response rate (ORR) assessed by the independent imaging evaluation committee was confirmed to be as high as 73.1%, DCR was 92.3%, median progression free survival (mPFS) was 9.3 months, PFS rate at 9 months was 63.7%, and 1-year OS rate was 83.1%. Treatment response was observed in all assessable subgroups, including patients with brain metastases and patients with different EGFR Ex20ins mutation subtypes.
Safety analysis: A total of 108 patients were included in the safety analysis set. During the dose escalation Phase, no dose limiting toxic reactions (DLT) occurred and the maximum tolerable dose (MTD) was not reached. 107 cases (99.1%) and 41 cases (38.0%) of patients experienced any level and ≥ 3 levels of treatment emergent adverse events (TEAEs), respectively; 102 (94.4%) and 30 (27.8%) patients experienced treatment-related adverse events (TRAEs) of any level and ≥ 3 level, respectively. The most common TEAEs are diarrhea (46.3%), anemia (38.0%), and rash (32.4%).
The above results indicate that YK-029A has good tolerance and safety, and shows preliminary excellent therapeutic potential in NSCLC patients with EGFR Ex20ins mutation at initial treatment.


[Professor Duan Jianchun from the Cancer Hospital of the Chinese Academy of Medical Sciences stated in an interview at the ASCO site that]
The preferred first-line treatment for EGFR Ex20ins mutation is still based on the treatment plan for advanced NSCLC with negative driver genes, with chemotherapy or chemotherapy combined with immunotherapy or chemotherapy combined with angiogenesis as the first-line recommendation. For patients with EGFR Ex20ins mutation, traditional treatment methods show limited effects, and clinical needs have not been met yet. YK-029A has been found in preclinical animal trials and early clinical studies to not only target T790M mutations, but also some non-common EGFR mutations, such as Ex20ins mutations, showing excellent therapeutic effects. YK-029A demonstrated excellent clinical efficacy and good drug safety and tolerability in the initial treatment of Ex20ins mutation cohort. The IRC assessed ORR exceeded 70% and PFS exceeded 9 months, while the effective rate in traditional chemotherapy or chemotherapy combined with immunotherapy was only about 30% -40%, and PFS was only 6 months. This new treatment option has a larger scale and greater degree of extension in effective rate and median PFS, and YK-029A is expected to have good study prospects and treatment effects for Ex20ins mutation patients.

[Guo Yongqi, CEO of Puhe Biopharma, stated]
YK-029A is a Class I innovative drug originally developed in China, targeting EGFR ex20ins mutations and EGFR sensitive mutations in advanced NSCLC. The Phase I clinical study results of YK-029A have been updated again with the latest study data following last year's CSCO debut, and have been included in the poster discussion at this year's ASCO Meeting, reflecting the high recognition of YK-029A by the international academic community. Puhe Biopharma will continuously innovate in multiple tumor and related treatment fields such as lung cancer, with the goal of producing more internationally recognized breakthrough innovative drugs. We look forward to our innovative achievements benefiting patients worldwide as soon as possible.

About YK-029A
YK-029A is an oral, irreversible, highly selective EGFR tyrosine kinase inhibitor (TKI) targeting multiple EGFR mutation subtypes. With excellent efficacy and safety, it was designated as a breakthrough therapy (BTD) in China in 2022, becoming the first breakthrough therapeutic drug in the field of EGFR Ex20ins lung cancer for initial treatment, the first indication for YK-029A is "first-line treatment of EGFR Ex20ins mutation NSCLC", which is currently in the Phase III registration clinical enrollment stage nationwide and has been studied in 60 centers nationwide.
About Suzhou Puhe Biopharma Co., Ltd.
Puhe Biopharma is a small molecule innovative pharmaceutical company jointly funded and incubated by Shiyu Capital and well-known domestic listed pharmaceutical companies. Our focus is on developing groundbreaking drugs in the field of oncology and supportive cancer care, committed to addressing distinct clinical needs. We adopt a multifaceted approach that blends scientific insight, financial acumen, and industrial strategy, alongside a market-oriented, risk-balanced operational method.
Leveraging our domestic clinical strengths through strategies such as company mergers and acquisitions, product introductions, and independent R&D, Puhe Biopharma actively explores commercial opportunities. We capitalize on the benefits of flexible production scale and comparatively low-cost investments, integrating resources to expedite our industrial growth. In 2022, our innovative approach earned us a spot in the Jiangsu Province Science and Technology Small and Medium-Sized Enterprise Database.
Currently, Puhe Biopharma has an impressive portfolio of small molecule drugs for cancer and supportive therapies, targeting unmet clinical needs in lung and pancreatic cancer, among others. A standout in our product pipeline is YK-029A, a leading and rapidly advancing innovative drug. Having received acceptance and approval from the National Medical Products Administration, YK-029A is now progressing into the pivotal Phase III of clinical trials.